Exploring Flavonoid Effects on Respiratory Epithelium: Insights Using the EasyMount Ussing Chamber
In the study “Modulation of the Respiratory Epithelium Physiology by Flavonoids—Insights from 16HBEσ Cell Model,” researchers actively examined how specific flavonoids—kaempferol, luteolin, as well as naringenin—effect the physiology of human bronchial epithelial cells. Researchers sought to understand how these compounds importantly influence multiple aspects of cell behavior. They concentrated on cell metabolic activity, proliferation, epithelial barrier integrity and ion transport mechanisms with particular emphasis on the cystic fibrosis transmembrane conductance regulator (CFTR) channel.
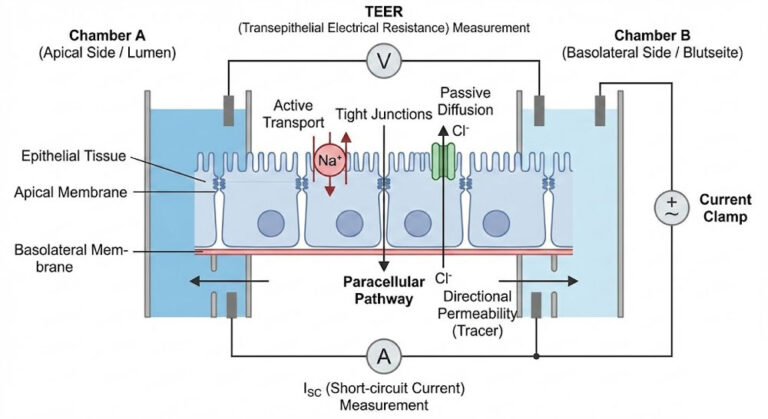
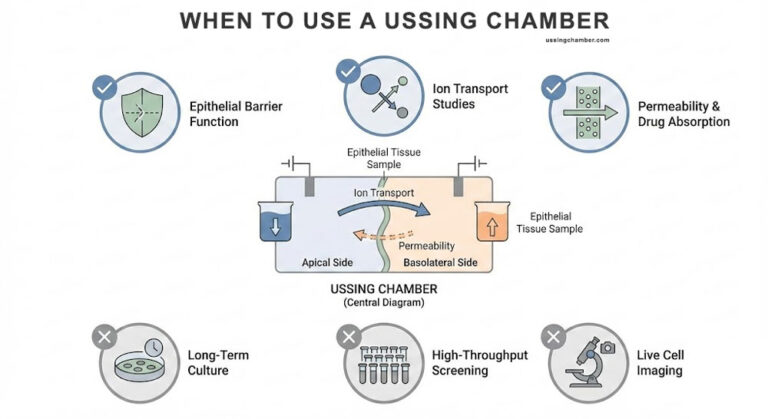
The team carefully used the EasyMount Ussing Chamber system from Physiologic Instruments to actively assess the effect of flavonoids on ion transport. This apparatus enabled researchers to mount polarized cell monolayers of 16HBE14σ cells between two chambers, thereby creating special apical as well as basolateral compartments. They applied a chloride gradient across the monolayer. By measuring the short-circuit current (Isc) under voltage-clamp conditions, they could evaluate CFTR-mediated chloride transport. Researchers conducted experiments on luteolin-treated cells at 50 μM for 72 hours. They also tested untreated cells to determine the flavonoid’s effect on chloride secretion.
Luteolin treatment importantly increased CFTR-mediated chloride transport, as shown by elevated Isc values, according to the Ussing Chamber measurements. It is suggested that the activity of CFTR channels is improved by luteolin, which potentially leads to an improvement in the movement of chloride ions across the epithelial barrier. The modulation of ion transport is considered important for maintaining proper respiratory epithelial function, with possible therapeutic implications in conditions like cystic fibrosis, where CFTR function is compromised.
The study actively assessed the effects of the flavonoids on epithelial barrier integrity. It measured multiple levels of transepithelial electrical resistance (TER). All tested flavonoids improved epithelial integrity, with naringenin as well as luteolin showing the most important effect. This enhancement of barrier function shows that these compounds may increase the epithelial defense against ecological insults as well as pathogens.
The Ussing Chamber system played an important role in clearly showing that luteolin importantly improves CFTR-mediated chloride transport in many human bronchial epithelial cells. The study offers costly understandings into how specific flavonoids can directly influence respiratory epithelial physiology, concentrating on their potential as therapeutic agents for improving epithelial barrier function as well as ion transport in respiratory diseases.
Article link: Modulation of the Respiratory Epithelium Physiology by Flavonoids—Insights from 16HBEσcell Model
Summary of the Abstract
Compromised airway epithelial barrier function is strongly associated with the development of numerous diseases, significantly contributing to global morbidity and mortality. Flavonoids, natural compounds known for their antioxidant and anti-inflammatory properties, have long been utilized for health benefits. Recent research has highlighted their potential to enhance airway epithelial barrier integrity. This study aimed to evaluate the effects of three flavonoids—kaempferol (flavonol), luteolin (flavone), and naringenin (flavanone)—on the integrity and functionality of the airway epithelial barrier using a human bronchial epithelial cell line (16HBE14σ). Researchers assessed their impact on transepithelial electrical resistance (TEER), ionic currents, cell migration, and proliferation through assays like MTT, trypan blue staining, wound healing, and Ussing chamber measurements.
The findings revealed that all three flavonoids were non-cytotoxic to 16HBE14σ cells at micromolar concentrations. While luteolin and naringenin increased cell metabolic activity significantly, naringenin had no effect on cell proliferation, and both kaempferol and luteolin exhibited inhibitory effects. TEER measurements demonstrated that all tested flavonoids improved epithelial barrier integrity, with naringenin and luteolin showing the most substantial enhancements. Importantly, Ussing chamber experiments indicated that luteolin increased chloride ion transport via the cystic fibrosis transmembrane conductance regulator (CFTR) channel, highlighting its potential impact on ion transport regulation.
This study underscores the nuanced effects of flavonoids on airway epithelial cells, showing that despite their similar chemical structures, they exert distinct influences on cellular processes. Kaempferol, luteolin, and naringenin varied in their ability to modulate metabolic activity, proliferation, and barrier function. These findings contribute valuable insights into the therapeutic potential of flavonoids in respiratory health, offering a foundation for further exploration of their applications in enhancing epithelial integrity and managing respiratory diseases.
What is the cystic fibrosis transmembrane conductance regulator (CFTR) channel?
The role of regulating ion as well as fluid transport across epithelial membranes is played by the cystic fibrosis transmembrane conductance regulator (CFTR) channel, which is a specialized protein. It belongs to the ATP-binding cassette (ABC) transporter family, functioning mainly as both a chloride ion channel along with other roles. CFTR plays an important role in maintaining a proper balance of salt and water. It is necessary for surfaces like those found in the lungs, intestines, pancreas and sweat glands.
Key Features of CFTR:
- Structure:
- CFTR is a large, transmembrane protein with two nucleotide-binding domains (NBDs) that bind and hydrolyze ATP.
- It also has two membrane-spanning domains (MSDs) that form the channel through which chloride ions pass.
- A unique regulatory (R) domain, phosphorylated by protein kinase A (PKA), modulates the opening and closing of the channel.
- Function:
- The CFTR channel transports chloride ions (Cl⁻) across epithelial cell membranes, contributing to the movement of water by osmosis.
- This process is vital for producing thin, freely flowing mucus and maintaining hydration of epithelial surfaces.
- CFTR also indirectly regulates other channels, including sodium (ENaC) and potassium channels, further affecting ion homeostasis.
- Regulation:
- CFTR is tightly regulated by ATP binding and hydrolysis, as well as by phosphorylation of its R domain.
- Activation typically occurs in response to signals like cAMP, which activate PKA to phosphorylate the R domain, opening the channel.
CFTR and Cystic Fibrosis:
Mutations in the CFTR gene can impair the production, folding, or function of the protein, leading to cystic fibrosis (CF). CF is a genetic disorder characterized by:
- Thick, sticky mucus in the lungs, causing respiratory complications.
- Blockage of pancreatic ducts, affecting digestion.
- Elevated salt levels in sweat.
The most common CFTR mutation, ΔF508, results in a misfolded protein that is degraded before reaching the cell membrane. Other mutations may affect the channel’s function, stability, or regulation.
CFTR in Research and Therapeutics:
CFTR is a focus of extensive research due to its role in cystic fibrosis and other conditions affecting epithelial ion transport. Therapies targeting CFTR include:
- Potentiators (e.g., Ivacaftor): Improve the function of existing CFTR channels.
- Correctors (e.g., Lumacaftor): Help misfolded CFTR reach the membrane.
- Amplifiers: Increase CFTR protein production.
Beyond CF, CFTR is studied in contexts like diarrhea caused by overactive CFTR and its involvement in regulating epithelial barrier functions. Understanding its mechanisms is vital for developing targeted treatments for a range of conditions.