The Ussing Chamber
A sophisticated laboratory tool known as an Ussing Chamber actively investigates the transport of ions, nutrients, as well as drugs across epithelial tissues. Hans Ussing developed this device in the 1950s. It serves as a necessary tool in physiology as well as pharmacology. Researchers can analyze the functional properties of epithelial barriers in a controlled environment. The Ussing Chamber enables researchers to precisely measure multiple active and passive transport processes, including “ion flux,” “permeability,” and “electrical resistance,” by isolating a specific segment of epithelial tissue and positioning it between two compartments. These impressive capabilities truly make it indispensable for effectively studying multiple phenomena, such as nutrient absorption in the intestine, drug permeability in pharmacological testing, as well as epithelial barrier dysfunction in disease models.
Offering impressive flexibility, the ussing chamber can simulate physiological conditions as well as provide quantitative, real-time data. By employing specialized electrodes along with solutions, it delivers costly understandings into transepithelial electrical properties, including short-circuit current (Isc) as well as transepithelial resistance (TER), which serve as indicators of ion transport activity and barrier integrity, respectively. By manipulating the experimental conditions, such as ion gradients or drug concentrations, researchers can explore specific mechanisms of transport or drug action. The Ussing Chamber has been positioned as a basis in advancing comprehension of epithelial physiology, as well as its applications in drug development along with disease research, due to this adaptability.
History of Ussing Chambers
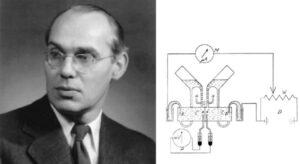
In the early 1950s Ussing played an innovating role in explaining how ions actively transport across frog skin. He was the first to describe this mechanism. He actively studied biology as well as geography at the University of Copenhagen. In 1943, he received his master’s degree, graduating with many honors. The Amory Prize of the American Academy of Arts and Sciences was awarded to him in 1970.
Beginning his scientific career, Hans H. Ussing, as a passionate zoologist, energetically studied the diverse marine plankton fauna in East Greenland. He came into contact with August Krogh while George de Hevesy, Niels Bohr and Krogh planned to apply artificial radioactive isotopes. They targeted to study the dynamic state of the living organism. Ussing boldly initiated a revolutionary new era of studies on transport across epithelial membranes. This followed his important studies of protein turnover of body tissues using deuterium-labeled amino acids. Novel concepts such as exchange diffusion, unidirectional fluxes, flux-ratio equation, as well as solvent drag were resulted from theoretical difficulties in interpreting tracer fluxes.
Proving The Active Transport Of Sodium Ions
By combining methods of biophysics with radioactive isotope technology, Ussing importantly introduced and defined the phrases ‘short-circuit current’, ‘active transport pathway’, as well as ‘shunt pathway’ and with frog skin serving as the experimental model, he descriptively proved the active transport of sodium ions. Ussing theorized that the transepithelial ion fluxes importantly related to the bioelectric potentials that were previously puzzling. He conceived this idea based on his electric circuit analogue of frog skin. The two-membrane hypothesis of frog skin ignited the study of epithelial transport at the cellular level, which raised new questions regarding the cellular mechanisms of actions of hormones as well as drugs. His theoretical treatment of osmotic water fluxes along with fluxes of deuterium labeled water led to the important discovery of multiple epithelial water channels.
His outstanding discovery of paracellular transport in frog skin effectively bridged studies of high as well as low resistance epithelia and widely generalized the description of epithelial transport. The last decade of his scientific life was devoted by him to solute-coupled water transport, as well as multiple related studies. He presented the sodium recirculation theory of isotonic transport, along with clearly obtaining evidence for the recirculation of sodium ions in a toad’s small intestine through an experimental study. Ussing conducted penetrating analyses of important aspects of epithelial membrane transport, offering understandings of general applicability along with powerful analytical methods for studying the intestine, kidney, respiratory epithelia, as well as exocrine glands—each of equal importance to biology and medicine.
Solute Transport
Researchers employ an Ussing chamber to thoroughly study solute transport across a confluent layer of cells. Typically, a monolayer of epithelial cells creates a barrier between the external as well as internal environments of the body, such as those that line the airways, intestinal tract, along with the bladder. However, it can also consist of a multilayered structure like skin, 3D cell culture, or a confluent layer of endothelial cells, such as the blood-brain barrier in culture or corneal endothelium. Researchers can determine solute transport by measuring the solute’s flux or, in cases involving charged solutes like ions, by measuring the current across the tissue. Ion transport and tissue integrity are assessed by these electrical parameters, along with tissue resistance.
To achieve these measurements, three essential components are carefully designed in Ussing systems: the “Ussing chamber,” the “voltage and current clamp,” and a “data acquisition system.” The Ussing chamber allows researchers to place tissue in solution-filled compartments, where electrodes can be installed to measure voltage and pass current through the tissue. The voltage current clamp actively measures either voltage or current across the epithelium, enabling determination of bioelectrical properties such as transepithelial voltage, short-circuit current, resistance, or conductance. Researchers can record electrical measurements using simple chart recorders or advanced electronic systems, with modern data acquisition setups actively collecting, measuring, and plotting tissue resistance or conductance in real time.
Commonly asked questions about Ussing Chambers
What is an Ussing Chamber?
Answer. Researchers frequently use an Ussing Chamber to effectively measure a variety of transport processes, including large movement of ions, nutrients and drugs across epithelial tissues. This setup allows researchers to carefully study the electrophysiological properties and permeability of biological membranes in a controlled environment.
What are the main applications of an Ussing Chamber?
Answer. Common applications include:
- Analysis of Active and Passive Transport: The Ussing Chamber is widely used to study the movement of ions like sodium (Na⁺), chloride (Cl⁻), and potassium (K⁺) across epithelial tissues.
- Electrophysiological Measurements: Parameters such as short-circuit current (Isc) and transepithelial resistance (TER) are measured to investigate transport mechanisms and channel functionality, including the activity of the cystic fibrosis transmembrane conductance regulator (CFTR) in diseases like cystic fibrosis.
2. Epithelial Barrier Function Assessment:
- Transepithelial Resistance (TER): The chamber is instrumental in evaluating the integrity of the epithelial barrier, identifying disruptions or enhancements in barrier function.
- Permeability Testing: Researchers use the Ussing Chamber to measure the permeability of epithelial layers to ions, drugs, and other molecules.
3. Nutrient Absorption:
- Gastrointestinal Research: The chamber is used to investigate the transport and absorption of nutrients, such as glucose, amino acids, and vitamins, across intestinal epithelial cells.
- Malabsorption Studies: It aids in understanding conditions like celiac disease or inflammatory bowel disease (IBD) that affect nutrient uptake.
4. Drug Development and Permeability Testing:
- Drug Absorption: The Ussing Chamber is employed to evaluate the permeability of epithelial tissues to new drug candidates, aiding in predicting oral bioavailability.
- Pharmacological Testing: Researchers study the effects of drugs on ion channels, transporters, and epithelial barrier function to identify potential therapeutic applications.
5. Disease Modeling:
- Cystic Fibrosis: The chamber is frequently used to study the defective chloride transport in epithelial tissues from cystic fibrosis models.
- Inflammatory Diseases: It helps explore the pathophysiology of diseases like IBD by simulating inflammatory conditions and assessing their impact on barrier function and ion transport.
6. Effects of Bioactive Compounds:
- Nutritional Research – It evaluates how dietary components, such as flavonoids, bile acids, and probiotics, influence ion transport and epithelial integrity.
- Toxicology Studies: The chamber helps determine the impact of environmental toxins or pharmaceutical compounds on epithelial health and function.
7. Comparative Physiology
- Animal Models: It is used to compare epithelial transport and barrier properties across different species, offering insights into evolutionary adaptations and interspecies differences.
How does an Ussing Chamber work?
Answer. The Ussing Chamber separates two compartments while containing an epithelial tissue sample. Electrodes actively measure necessary electrical properties such as short-circuit current (Isc) as well as transepithelial resistance (TER). Researchers can assess transport activity across the tissue. They use solutions containing specific ions or compounds to do this.
What types of tissues can be used in an Ussing Chamber?
Answer. Ussing Chambers are suitable for various epithelial tissues, such as:
- Intestinal mucosa.
- Lung epithelium.
- Skin.
- Bladder or kidney epithelium.
What measurements can be obtained from an Ussing Chamber?
Answer. An Ussing Chamber allows researchers to measure various physiological and biophysical properties of epithelial tissues. Common measurements obtained include:
1. Short-Circuit Current (Isc):
- Measures ion transport across the epithelial layer.
- Indicates active transport processes like sodium absorption or chloride secretion.
2.Transepithelial Electrical Resistance (TEER):
- Assesses the integrity and permeability of the epithelial barrier.
- Higher TEER indicates tighter barriers, while lower TEER suggests increased permeability.
3. Ion Flux:
- Quantifies the movement of specific ions (e.g., Na⁺, Cl⁻) across the tissue.
- Used to study active and passive transport mechanisms.
4. Electrical Potential Difference (PD):
- Measures the voltage difference across the epithelial tissue.
- Provides insight into the electrochemical gradients.
5. Transport Rates of Nutrients or Drugs:
- Evaluates the permeability and absorption of substances like glucose, amino acids, or pharmaceutical compounds.
6. Barrier Function Studies:
- Measures the passage of macromolecules or tracers to evaluate barrier integrity and potential disruptions.
7. pH and Ion Concentration Changes:
- Monitors alterations in pH or specific ion concentrations in the chambers, reflecting transport activity.
8. Metabolic Activity:
- Assesses the tissue’s metabolic responses during transport processes.
These measurements are critical for studying epithelial physiology, drug absorption, nutrient transport, and disease models involving epithelial dysfunction.
How do you prepare tissues for an Ussing Chamber?
Answer. Tissue preparation involves:
- Isolating the epithelium layer carefully to avoid damage.
- Mounting it between the chamber’s compartments.
- Keeping the tissue hydrated with physiological solutions until use.
What solutions are used in the Ussing Chamber?
Answer. Typically, Ringer’s solution or another ion-specific buffer is used. These solutions can be modified to include drugs, nutrients, or other test compounds to study their transport effects.
What are the advantages of using an Ussing Chamber?
Answer. Ussing chambers provides:
- Real-time, quantitative data on transport mechanisms.
- A controlled environment for specific experimental conditions.
- Insights into both active and passive transport processes.
What challenges are commonly faced when using Ussing Chambers?
Answer. Some common challenges researchers face with Ussing chambers include:
- Tissue viability over time.
- Maintaining a leak-free seal around the tissue.
- Ensuring electrode calibration for accurate measurements.
How do you maintain and clean an Ussing Chamber?
Answer. Maintaining a Ussing Chamber can be simple. Make sure to:
- Rinse all components with distilled water after use.
- Periodically clean electrodes with a mild detergent or specific cleaning solutions.
- Check seals and O-rings for wear and replace them as needed.
- Regularly calibrate the electrodes and validate the system.
Overall, researchers widely consider the Ussing Chamber to be a highly effective and flexible tool for effectively examining the transport of many ions, nutrients and drugs across multiple epithelial tissues. A controlled and quantitative platform is provided. Researchers are enabled to explore important physiological and pharmacological processes such as ion transport, epithelial barrier integrity and drug permeability. The answers to these frequently asked questions effectively present the chamber’s wide-ranging applications, from basic science research to drug development, as well as its capability to provide real-time understandings into complicated biological mechanisms.
The Ussing Chamber demands careful preparation as well as consistent maintenance, and precise calibration for accurate results. However, its benefits truly outweigh these challenges. Nutrient absorption is studied and new pharmaceuticals are tested by using this device. The investigation of disease-related epithelial dysfunction is also supported by this indispensable tool in advancing comprehension of epithelial transport, and its implications in health and disease.
